Santonin Source, Use and Structure – Complete Notes By Sadre Alam
Source of Santonin
It is obtained from the dried expended flower heads of Artomision Ciua (Wormseed)
Use:-
- Due to toxicity it is now replaced by other an the limintics.
- It is mostly used an anthelmintic
- It is very efficient in this action on round worms. E.g.(Ascaris) in does of 60 to 200mg daily for 3day; but shows less effect on the these worms and none on taenia.
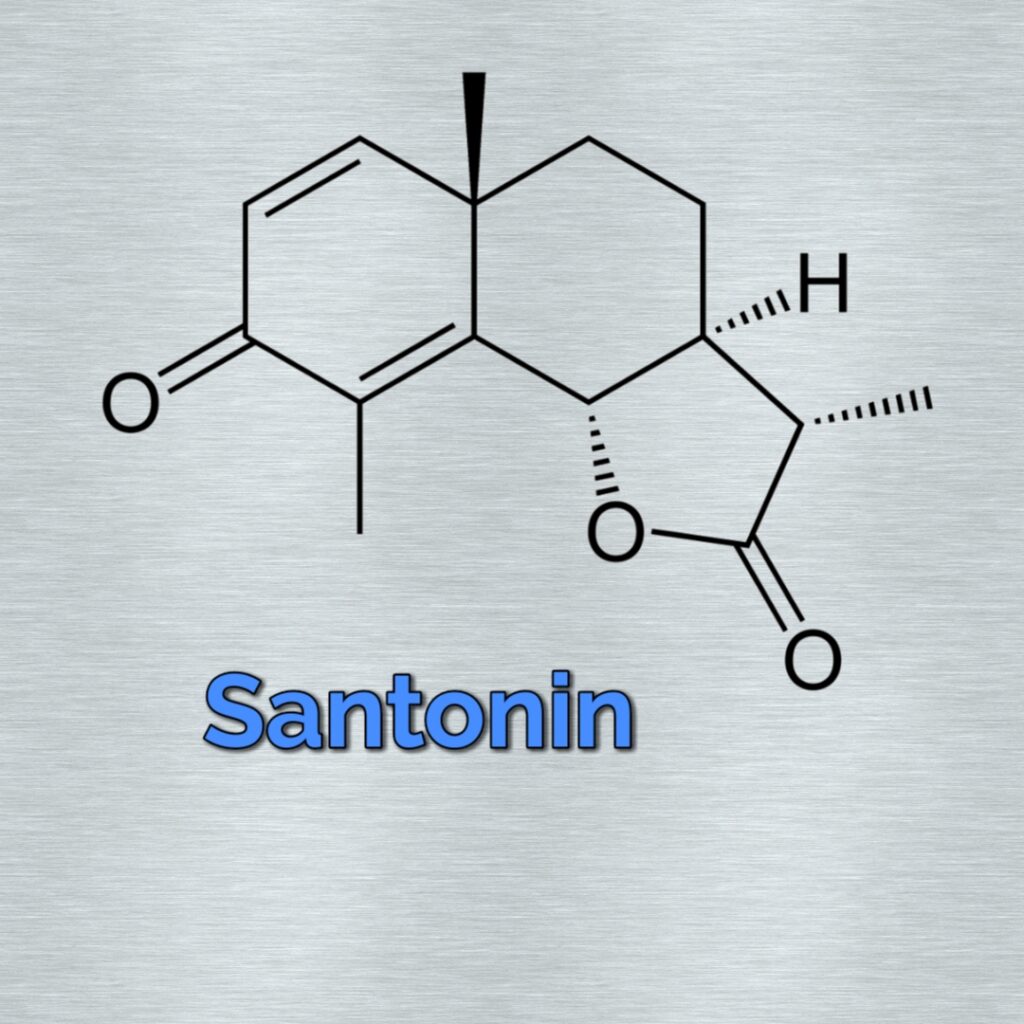
Structure
- Molecular Formula of santonin (C₁₅H₁₈O)
- Santonin belongs to sesquiterpenoid class of tere noida.
- It contains three isoprene units joined head to tail.
DBE=X+1-Y/Z
=16-9
=07 【CxHyOz】
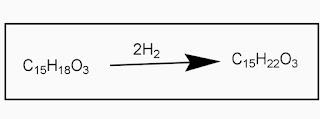
- Hydrogenation of santonin forms tetrahydrosantonin.
Santon in Contains two C=C double bond (DBE=O2)
Santonin Forms Oxime Derivative With NH2OH
Santonin contains carbon group and it was found to be ketonic (DBE=01)
This ketonic behaves as α,β-ke rone. This proved from the UV-Specturm of santonin.


Santonin
C15H18O3 | Sesquiterpene Lactone | Anthelmintic Agent

Introduction
Santonin is a bioactive sesquiterpene lactone originally derived from the unexpanded flower heads of Artemisia cina (Levant wormseed) and related Artemisia species. Historically used as an anthelmintic, its clinical use has declined due to toxicity concerns, though it remains an important compound in phytochemical research.
Chemical Properties
Structural Characteristics
Key Structural Features:
- Sesquiterpenoid with three isoprene units (C15 compound)
- Contains a γ-lactone ring (cyclic ester)
- α,β-unsaturated ketone moiety (C=O conjugated with C=C)
- Tricyclic system with decalin core
- Three chiral centers (3S, 3aR, 9aS configuration)
Double Bond Equivalent (DBE) Analysis:
DBE = C – (H/2) + (N/2) + 1 = 15 – (18/2) + 0 + 1 = 7
This accounts for:
- 1 carbonyl group (C=O)
- 2 carbon-carbon double bonds (C=C)
- 4 rings (tricyclic system + lactone)
Pharmacological Activity
⚠️ Toxicity Note
Santonin has a narrow therapeutic index and can cause serious adverse effects including xanthopsia (yellow vision), neurotoxicity, and gastrointestinal disturbances. Modern anthelmintics are preferred.
Primary Actions:
- Anthelmintic: Particularly effective against Ascaris lumbricoides (roundworm)
- Dosage: 60-200 mg daily for 3 days (historical use)
- Mechanism: Paralyses worms by interfering with their energy metabolism
Spectrum of Activity:
| Organism | Sensitivity |
|---|---|
| Ascaris lumbricoides (roundworm) | High |
| Hookworms | Moderate |
| Threadworms | Low |
| Taenia species (tapeworms) | None |
Characteristic Reactions
Hydrogenation
Catalytic hydrogenation reduces the double bonds to form tetrahydrosantonin:
C15H18O3 + 2H2 → C15H22O3
Oxime Formation
Reaction with hydroxylamine confirms the ketone functionality:
C15H18O3 + NH2OH → C15H18O3=NOH + H2O
UV-Vis Spectroscopy
Shows characteristic absorption of α,β-unsaturated ketone at λmax ≈ 240-250 nm (ε ≈ 10,000)
Biosynthesis
Santonin is biosynthesized in Artemisia species via the mevalonate pathway:
- Formation of farnesyl pyrophosphate (FPP) from isoprene units
- Cyclization to form germacrene A
- Oxidation and rearrangement to form the santonin skeleton
- Final oxidative modifications to yield santonin










Post Comment