How to Citral isolation from Natural Sources, Chemical Structure and Synthesis Methods, Use And Objective questions and answers
Citral
Citral is widely distributed and occurs to the extent of 70 to 80 percent in lemongrass oil.
IsolationThe essential oil containing citral is treated With sodium bisulphite solution,when crystalline citral bisulphite derivative is obtained. This derivative is then hydrolyzed with sodium carbonate to give pure citral.
Chemical Structure of Citral
- Molecular Formula: C₁₀H₁₆O (monoterpene aldehyde).
- Isomers
- Geranial (trans-citral, E-isomer) – Strong lemon odor.
- Neral (cis-citral, Z-isomer) – Milder, sweeter aroma.
- Functional Group: α,β-unsaturated aldehyde (reactive site).
- Spectroscopic Data:
- IR: Strong peak at ~1680 cm⁻¹ (C=O stretch).
- NMR: Characteristic aldehydic proton at ~9-10 ppm.
Structure of Citral
- Citral react with hydroxylamine to form an Oxime.

- Citral reacts with bromine to form a tetrabromide dederivative.

This indicated the presence of two C-C double bonds in the citral.
- Citral undergoes hydrolysis with potassium carbonate to give 2-methyl-2-hepten-6-one(Methylheptenone) and acetaldehyde.

Synthesis Of Citral (Barbier-Bouveaul-Tiemann’s)
Citral can be synthesis form 2-methyl-2-hepten-6-oneby the following three steps.
Step1:- Methyheptenone is subject to reformatsky reaction and hydrolysis to give a β-hydroxy acid.
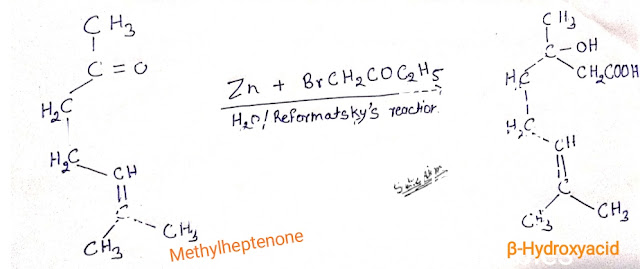
Step2:- Dehydration of β-hydroxy acid with anhydride give geranic acid.

Step3:- Distillation of a mixture containing the calcium salts of geranic acid and calcium formate yields citral

- Determination
- Inorganic Chemistry
- Normality
- Organic Chemistry
- Physical Chemistry
- Practical
- Reaction
- Uncategorized
Use Of Citral
citral is a natural occuring aromatic compound fragrance in perfume, soaps, and air fresheners.
Its use a flavoring agent in soft drinks, candies, bakery products and other foods item in small and safe amount.
Its added to creams, lotions, shampoos and cosmetics to provide a pleasant fragrance.
Citral is use in some madecine and mild antimicrobial properties.
Due to the strong smell used in mosquito repellents and insect-repelling products.
Short Exam Question and Answers
Write one use of citral?
Ans– Citral is used in the manufacture of Vitamin A.
Why citral use in Perfumes?
Ans-Citral is used in perfumes because it has a pleasant lemon Fragrance.
Citral natrula or synthetic?
Ans– Citral is a naturally compound, though it can also be prepared synthetically.
Why is citral used in insect repellents?
Ans– Citral has a strong odour that helps repel insects.
- Carbon and Its Compounds 50 MCQs with Answers | Class 10 Chemistry by Sadre Alam
- Acids, Bases and Salts Class 10 MCQs (20 Questions)
- Class 10 Chemistry Chemical Reaction MCQs Notes and Test
- Metals and Non-Metals Class 10 – MCQ Questions
- Chlorine Position in Periodic Table, Occurrence, Preparation, Reaction and Uses Example












1 comment