How to learn Electronic Configurations of All Elements
Electronic configuration depending on s, p, d and f orbital.
S-Block
Subshell– S-orbitals 2 electron
Group– Alkali Metals and alkaline earth metals
Configuration– ns1 (Group 1) and ns2 ( Group 2 ), Where n = Energy level
P-Block
Subshell– p-orbitals 6 electron
Group– 13 to 18 group
Configuration– ns2np1-6
d-Block
Subshell– d-orbitals 10 electron
Group– 3 to 12 group (Transition Metals)
Configuration– (n-1)d1-10 s1-2
f-Block
Subshell– d-orbitals 14 electron
Group- Lanthanides(Z 58 to 71) and Actinides (Z 90 to 103)
Configuration-(n-1)f1-14 (n-1)d1-10 s1-2
Principles for Electron Configurations
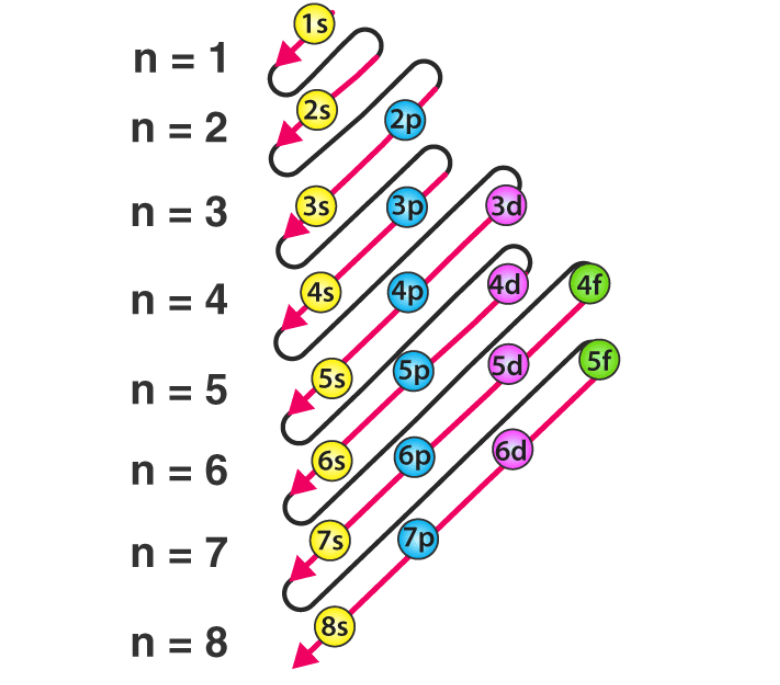
Aufbau Principle– Electrons fill orbitals from lowest to highest energy (1s → 2s → 2p → 3s → 3p → 3d → 4s, etc.).
Pauli Exclusion Principle: Each orbital holds up to 2 electrons with opposite spins.
Hund’s Rule: Electrons fill degenerate orbitals (e.g., p, d, f) singly with parallel spins before pairing.
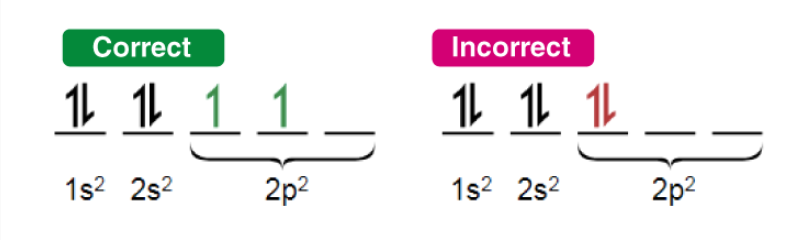
Exceptions Elements like Cr, Cu, Mo, and some f-block elements deviate for stability (half-filled or fully filled subshells).
| Atomic Number | Symbol | Element Name | Electronic Configuration |
|---|---|---|---|
| 1 | H | Hydrogen | 1s1 |
| 2 | He | Helium | 1s2 |
| 3 | Li | Lithium | 1s2 2s1 OR [He] 2s1 |
| 4 | Be | Beryllium | 1s2 2s2 OR [He] 2s2 |
| 5 | B | Boron | 1s2 2s2 2p1 OR [He] 2s2 2p1 |
| 6 | C | Carbon | 1s2 2s2 2p2 OR[He] 2s2 2p2 |
| 7 | N | Nitrogen | 1s2 2s2 2p3 OR[He] 2s2 2p3 |
| 8 | O | Oxygen | 1s2 2s2 2p4 OR [He] 2s2 2p4 |
| 9 | F | Fluorine | 1s2 2s2 2p5 OR [He] 2s2 2p5 |
| 10 | Ne | Neon | 1s2 2s2 2p6 OR [He] 2s2 2p6 |
| 11 | Na | Sodium | 1s2 2s2 2p6 3s1 OR [Ne] 3s1 |
| 12 | Mg | Magnesium | 1s2 2s2 2p6 3s2 OR [Ne] 3s2 |
| 13 | Al | Aluminum | 1s2 2s2 2p6 3s2 3p1 OR [Ne] 3s2 3p1 |
| 14 | Si | Silicon | 1s2 2s2 2p6 3s2 3p2 OR [Ne] 3s2 3p2 |
| 15 | P | Phosphorus | 1s2 2s2 2p6 3s2 3p3 OR [Ne] 3s2 3p3 |
| 16 | S | Sulfur | 1s2 2s2 2p6 3s2 3p4 OR [Ne] 3s2 3p4 |
| 17 | Cl | Chlorine | 1s2 2s2 2p6 3s2 3p5 OR [Ne] 3s2 3p5 |
| 18 | Ar | Argon | 1s2 2s2 2p6 3s2 3p6 OR [Ne] 3s2 3p6 |
| 19 | K | Potassium | 1s2 2s2 2p6 3s2 3p6 4s1 OR [Ar] 4s1 |
| 20 | Ca | Calcium | 1s2 2s2 2p6 3s2 3p6 4s2 OR [Ar] 4s2 |
| 21 | Sc | Scandium | 1s2 2s2 2p6 3s2 3p6 3d1 4s2 OR [Ar] 3d1 4s2 |
| 22 | Ti | Titanium | 1s2 2s2 2p6 3s2 3p6 3d2 4s2 OR [Ar] 3d2 4s2 |
| 23 | V | Vanadium | 1s2 2s2 2p6 3s2 3p6 3d3 4s2 OR [Ar] 3d3 4s2 |
| 24 | Cr | Chromium | 1s2 2s2 2p6 3s2 3p6 3d5 4s1 OR [Ar] 3d5 4s1 |
| 25 | Mn | Manganese | 1s2 2s2 2p6 3s2 3p6 3d5 4s1 OR[Ar] 3d5 4s2 |
| 26 | Fe | Iron | 1s2 2s2 2p6 3s2 3p6 3d6 4s2 OR [Ar] 3d6 4s2 |
| 27 | Co | Cobalt | 1s2 2s2 2p6 3s2 3p6 3d7 4s2 OR [Ar] 3d7 4s2 |
| 28 | Ni | Nickel | 1s2 2s2 2p6 3s2 3p6 3d8 4s2 OR [Ar] 3d8 4s2 |
| 29 | Cu | Copper | 1s2 2s2 2p6 3s2 3p6 3d10 4s1 OR [Ar] 3d10 4s1 |
| 30 | Zn | Zinc | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 OR [Ar] 3d10 4s2 |
| 31 | Ga | Gallium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p1 OR [Ar] 3d10 4s2 4p1 |
| 32 | Ge | Germanium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2 OR [Ar] 3d10 4s2 4p2 |
| 33 | As | Arsenic | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p3 OR [Ar] 3d10 4s2 4p3 |
| 34 | Se | Selenium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p4 OR [Ar] 3d10 4s2 4p4 |
| 35 | Br | Bromine | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5 OR [Ar] 3d10 4s2 4p5 |
| 36 | Kr | Krypton | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 OR [Ar] 3d10 4s2 4p6 |
| 37 | Rb | Rubidium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s1OR [Kr] 5s1 |
| 38 | Sr | Strontium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2 OR [Kr] 5s2 |
| 39 | Y | Yttrium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d1 5s2 OR [Kr] 4d1 5s2 |
| 40 | Zr | Zirconium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d2 5s2 OR [Kr] 4d2 5s2 |
| 41 | Nb | Niobium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d4 5s1 OR [Kr] 4d4 5s1 |
| 42 | Mo | Molybdenum | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d5 5s1 OR [Kr] 4d5 5s1 |
| 43 | Tc | Technetium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d5 5s2 OR [Kr] 4d5 5s2 |
| 44 | Ru | Ruthenium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d7 5s1 OR [Kr] 4d7 5s1 |
| 45 | Rh | Rhodium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d8 5s1 OR [Kr] 4d8 5s1 |
| 46 | Pd | Palladium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 OR [Kr] 4d10 |
| 47 | Ag | Silver | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1 OR [Kr] 4d10 5s1 |
| 48 | Cd | Cadmium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 OR [Kr] 4d10 5s2 |
| 49 | In | Indium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p1 OR [Kr] 4d10 5s2 5p1 |
| 50 | Sn | Tin | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2 OR [Kr] 4d10 5s2 5p2 |
| 51 | Sb | Antimony | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p3 OR [Kr] 4d10 5s2 5p3 |
| 52 | Te | Tellurium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p4 OR [Kr] 4d10 5s2 5p4 |
| 53 | I | Iodine | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5OR [Kr] 4d10 5s2 5p5 |
| 54 | Xe | Xenon | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 OR [Kr] 4d10 5s2 5p6 |
| 55 | Cs | Cesium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s1 OR [Xe] 6s1 |
| 56 | Ba | Barium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2 OR [Xe] 6s2 |
| 57 | La | Lanthanum | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 5d1 6s2 OR [Xe] 5d1 6s2 |
| 58 | Ce | Cerium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f1 5d1 6s2 OR [Xe] 4f1 5d1 6s2 |
| 59 | Pr | Praseodymium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f3 6s2 OR [Xe] 4f3 6s2 |
| 60 | Nd | Neodymium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f4 6s2 OR [Xe] 4f4 6s2 |
| 61 | Pm | Promethium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f5 6s2 OR [Xe] 4f5 6s2 |
| 62 | Sm | Samarium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f6 6s2 OR [Xe] 4f6 6s2 |
| 63 | Eu | Europium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f7 6s2 OR [Xe] 4f7 6s2 |
| 64 | Gd | Gadolinium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f7 3d1 6s2 OR [Xe] 4f7 5d1 6s2 |
| 65 | Tb | Terbium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f9 6s2 OR [Xe] 4f9 6s2 |
| 66 | Dy | Dysprosium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f10 6s2 OR [Xe] 4f10 6s2 |
| 67 | Ho | Holmium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f11 6s2 OR [Xe] 4f11 6s2 |
| 68 | Er | Erbium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f12 6s2 OR [Xe] 4f12 6s2 |
| 69 | Tm | Thulium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f13 6s2 OR [Xe] 4f13 6s2 |
| 70 | Yb | Ytterbium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 6s2 OR [Xe] 4f14 6s2 |
| 71 | Lu | Lutetium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d1 6s2 OR [Xe] 4f14 5d1 6s2 |
| 72 | Hf | Hafnium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d2 6s2 OR [Xe] 4f14 5d2 6s2 |
| 73 | Ta | Tantalum | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d3 6s2 OR [Xe] 4f14 5d3 6s2 |
| 74 | W | Tungsten | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d4 6s2 OR [Xe] 4f14 5d4 6s2 |
| 75 | Re | Rhenium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d5 6s2 OR [Xe] 4f14 5d5 6s2 |
| 76 | Os | Osmium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d6 6s2 OR [Xe] 4f14 5d6 6s2 |
| 77 | Ir | Iridium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d7 6s2 OR [Xe] 4f14 5d7 6s2 |
| 78 | Pt | Platinum | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d9 6s1 OR [Xe] 4f14 5d9 6s1 |
| 79 | Au | Gold | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 OR [Xe] 4f14 5d10 6s1 |
| 80 | Hg | Mercury | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 OR [Xe] 4f14 5d10 6s2 |
| 81 | Tl | Thallium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p1 OR [Xe] 4f14 5d10 6s2 6p1 |
| 82 | Pb | Lead | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p2 OR [Xe] 4f14 5d10 6s2 6p2 |
| 83 | Bi | Bismuth | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p3 OR [Xe] 4f14 5d10 6s2 6p3 |
| 84 | Po | Polonium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p4 OR [Xe] 4f14 5d10 6s2 6p4 |
| 85 | At | Astatine | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p5 OR [Xe] 4f14 5d10 6s2 6p5 |
| 86 | Rn | Radon | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 OR [Xe] 4f14 5d10 6s2 6p6 |
| 87 | Fr | Francium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 7s1 OR [Rn] 7s1 |
| 88 | Ra | Radium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 7s2 OR [Rn] 7s2 |
| 89 | Ac | Actinium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 6d1 7s2 OR [Rn] 6d1 7s2 |
| 90 | Th | Thorium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 6d2 7s2 OR [Rn] 6d2 7s2 |
| 91 | Pa | Protactinium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f2 6d1 7s2 OR [Rn] 5f2 6d1 7s2 |
| 92 | U | Uranium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f3 6d1 7s2 OR [Rn] 5f3 6d1 7s2 |
| 93 | Np | Neptunium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f4 6d1 7s2 OR [Rn] 5f4 6d1 7s2 |
| 94 | Pu | Plutonium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f6 7s2 OR [Rn] 5f6 7s2 |
| 95 | Am | Americium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f7 7s2 OR [Rn] 5f7 7s2 |
| 96 | Cm | Curium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f7 6d1 7s2 OR [Rn] 5f7 6d1 7s2 |
| 97 | Bk | Berkelium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f9 7s2 OR [Rn] 5f9 7s2 |
| 98 | Cf | Californium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f10 7s2 OR [Rn] 5f10 7s2 |
| 99 | Es | Einsteinium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f11 7s2 OR [Rn] 5f11 7s2 |
| 100 | Fm | Fermium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f12 7s2 OR [Rn] 5f12 7s2 |
| 101 | Md | Mendelevium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f13 7s2 OR [Rn] 5f13 7s2 |
| 102 | No | Nobelium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 7s2 OR [Rn] 5f14 7s2 |
| 103 | Lr | Lawrencium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 7p1 7s2 OR [Rn] 5f14 7s2 7p1 |
| 104 | Rf | Rutherfordium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d2 7s2 OR [Rn] 5f14 6d2 7s2 |
| 105 | Db | Dubnium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d3 7s2 OR [Rn] 5f14 6d3 7s2 |
| 106 | Sg | Seaborgium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d4 7s2 OR [Rn] 5f14 6d4 7s2 |
| 107 | Bh | Bohrium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d5 7s2 OR [Rn] 5f14 6d5 7s2 |
| 108 | Hs | Hassium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d6 7s2 OR [Rn] 5f14 6d6 7s2 |
| 109 | Mt | Meitnerium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d7 7s2 OR [Rn] 5f14 6d7 7s2 |
| 110 | Ds | Darmstadtium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d8 7s2 OR [Rn] 5f14 6d8 7s2 |
| 111 | Rg | Roentgenium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d10 7s1 OR [Rn] 5f14 6d10 7s1 |
| 112 | Cn | Copernicium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d10 7s2 OR [Rn] 5f14 6d10 7s2 |
| 113 | Nh | Nihonium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d10 7s2 7p1 OR [Rn] 5f14 6d10 7s2 7p1 |
| 114 | Fl | Flerovium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d10 7s2 7p2 OR [Rn] 5f14 6d10 7s2 7p2 |
| 115 | Mc | Moscovium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d10 7s2 7p3 OR [Rn] 5f14 6d10 7s2 7p3 |
| 116 | Lv | Livermorium | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d10 7s2 7p4 OR [Rn] 5f14 6d10 7s2 7p4 |
| 117 | Ts | Tennessine | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d10 7s2 7p5 OR [Rn] 5f14 6d10 7s2 7p5 |
| 118 | Og | Oganesson | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f14 6d10 7s2 7p6 OR [Rn] 5f14 6d10 7s2 7p6 |
List the electron configurations of all the noble gases.
The electronic configurations of the noble gases are listed below.
Helium (He) – 1s2
Neon (Ne) – [He]2s22p6
Argon (Ar) – [Ne]3s23p6
Krypton (Kr) – [Ar]3d104s24p6
Xenon (Xe) – [Kr]4d105s25p6
Radon (Rn) – [Xe]4f145d106s26p6









6 comments