The Importance of the Periodic Table in Chemistry
The Importance of the Periodic Table in Chemistry
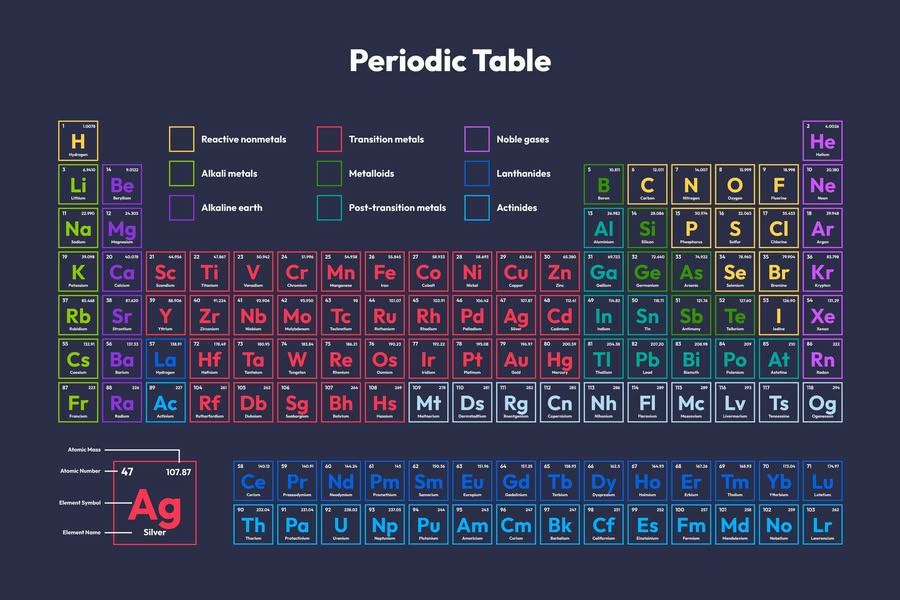
The periodic table is a systematic arrangement of all known chemical elements in order of their atomic number (number of protons in the nucleus) and their recurring chemical and physical properties. It is one of the most important tools in chemistry and provides a wealth of information about the elements and their relationships.
Periodic Table:
Elements: Each element is represented by its chemical symbol (e.g., H for hydrogen, O for oxygen) and is placed in a specific box with its atomic number and atomic mass.
Rows (Periods): The horizontal rows are called periods. There are 7 periods, each corresponding to the number of electron shells in an atom.
Columns (Groups):
The vertical columns are called groups or families. There are 18 groups, and elements in the same group share similar chemical properties due to having the same number of valence electrons.

Blocks:
The table is divided into blocks based on the subshell in which the last electron resides
s-block: Groups 1 and 2 (alkali and alkaline earth metals) plus hydrogen and helium.
p-block: Groups 13 to 18 (includes nonmetals, metalloids, and noble gases).
d-block: Groups 3 to 12 (transition metals).
f-block: Lanthanides and actinides (placed separately at the bottom).
Major Categories of Elements
Metals Found on the left and middle of the table. They are typically shiny, malleable, and good conductors of heat and electricity (e.g., iron (Fe), copper (Cu)).
Nonmetals:
Located on the upper right. They are generally poor conductors and can be gases, liquids, or solids (e.g., oxygen (O), sulfur (S)).
Metalloids:
Elements with properties intermediate between metals and nonmetals (e.g., silicon (Si), arsenic (As)).
Noble Gases:
Group 18 elements that are inert and non-reactive (e.g., helium (He), neon (Ne)).
Metals vs. Nonmetals vs. Noble Gases (Comparison Table)
| Property | Metals | Nonmetals | Noble Gases |
|---|---|---|---|
| Physical State | Solid (except Mercury) | Gas/Liquid/Solid (e.g., O₂, Br₂, S) | Gas (He, Ne, Ar, etc.) |
| Appearance | Shiny (Lustrous) | Dull (except Iodine) | Colorless, Odorless |
| Malleability | Malleable (can be hammered) | Brittle (breaks easily) | Not Applicable (Gaseous) |
| Ductility | Ductile (can be drawn into wires) | Non-ductile | Not Applicable |
| Conductivity | Good conductors of heat/electricity | Poor conductors (except Graphite) | Insulators (Poor conductors) |
| Reactivity | Reactive (lose electrons) | Reactive (gain electrons) | Non-reactive (Inert) |
| Electrons | Electropositive (donate e⁻) | Electronegative (accept e⁻) | Full valence shell (Stable) |
| Oxides | Basic oxides (e.g., CaO) | Acidic oxides (e.g., CO₂) | Do not form oxides |
| Examples | Iron (Fe), Copper (Cu), Gold (Au) | Carbon (C), Oxygen (O), Chlorine (Cl) | Helium (He), Neon (Ne), Argon (Ar) |
Importance of the Periodic Table
It helps predict the properties of elements and their behavior in chemical reactions.
It organizes elements in a way that highlights trends, such as atomic radius, electronegativity, and ionization energy.
It serves as a reference for scientists, students, and researchers in chemistry, physics, and related fields.
The periodic table was first developed by Dmitri Mendeleev in 1869, and it has since been refined as new elements have been discovered and our understanding of atomic structure has improved.
Let me know if you’d like to explore specific parts of the periodic table!
Electronic Configuration Trick
full electron configuration for elements 1 (Hydrogen) to 40 (Zirconium)
1. Hydrogen (H) – 1 electron
1s¹
2. Helium (He) – 2 electrons
1s²
3. Lithium (Li) – 3 electrons
1s² 2s¹
4. Beryllium (Be) – 4 electrons
1s² 2s²
5. Boron (B) – 5 electrons
1s² 2s² 2p¹
6. Carbon (C) – 6 electrons
1s² 2s² 2p²
7. Nitrogen (N) – 7 electrons
1s² 2s² 2p³
8. Oxygen (O) – 8 electrons
1s² 2s² 2p⁴
9. Fluorine (F) – 9 electrons
1s² 2s² 2p⁵
10. Neon (Ne) – 10 electrons
1s² 2s² 2p⁶
11. Sodium (Na) – 11 electrons
1s² 2s² 2p⁶ 3s¹
12. Magnesium (Mg) – 12 electrons
1s² 2s² 2p⁶ 3s²
13. Aluminum (Al) – 13 electrons
1s² 2s² 2p⁶ 3s² 3p¹
14. Silicon (Si) – 14 electrons
1s² 2s² 2p⁶ 3s² 3p²
15. Phosphorus (P) – 15 electrons
1s² 2s² 2p⁶ 3s² 3p³
16. Sulfur (S) – 16 electrons
1s² 2s² 2p⁶ 3s² 3p⁴
17. Chlorine (Cl) – 17 electrons
1s² 2s² 2p⁶ 3s² 3p⁵
18. Argon (Ar) – 18 electrons
1s² 2s² 2p⁶ 3s² 3p⁶
19. Potassium (K) – 19 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹
20. Calcium (Ca) – 20 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
21. Scandium (Sc) – 21 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹ 4s²
22. Titanium (Ti) – 22 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d² 4s²
23. Vanadium (V) – 23 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d³ 4s²
24. Chromium (Cr) – 24 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁵ 4s¹ (Exception)
25. Manganese (Mn) – 25 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁵ 4s²
26. Iron (Fe) – 26 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶ 4s²
27. Cobalt (Co) – 27 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁷ 4s²
28. Nickel (Ni) – 28 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁸ 4s²
29. Copper (Cu) – 29 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s¹ (Exception)
30. Zinc (Zn) – 30 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s²
31. Gallium (Ga) – 31 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p¹
32. Germanium (Ge) – 32 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p²
33. Arsenic (As) – 33 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p³
34. Selenium (Se) – 34 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁴
35. Bromine (Br) – 35 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁵
36. Krypton (Kr) – 36 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶
37. Rubidium (Rb) – 37 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 5s¹
38. Strontium (Sr) – 38 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 5s²
39. Yttrium (Y) – 39 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹ 5s²
40. Zirconium (Zr) – 40 electrons
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d² 5s²
- Exceptions:
- Chromium (Cr):
3d⁵ 4s¹(not3d⁴ 4s²) - Copper (Cu):
3d¹⁰ 4s¹(not3d⁹ 4s²)
(Due to half-filled/full-subshell stability)
- Chromium (Cr):
- Order of Filling:
Follows the Aufbau principle (1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d…). - Transition Metals (Sc-Zn):
Electrons fill the 3d orbital before the 4p orbital.









2 comments